The EU-CAD project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement 960899.
https://cordis.europa.eu/project/id/960899
“For MedTrace, the EU’s Horizon 2020 grant means we are able to complete our European pathway to market, to the benefits of patients and healthcare systems.” – Martin Stenfeldt, MedTrace, co-founder & CEO.
Eliminating Uncertainty in Coronary Artery Disease
CAD is the world’s leading cause of death, and healthcare systems all over the world rely on medical imaging tools for myocardial blood flow assessment with only 60% diagnostic sensitivity (accuracy). MedTrace’s innovation introduces a diagnostic solution that increases accuracy to 90% while increasing patient throughput 4-fold.
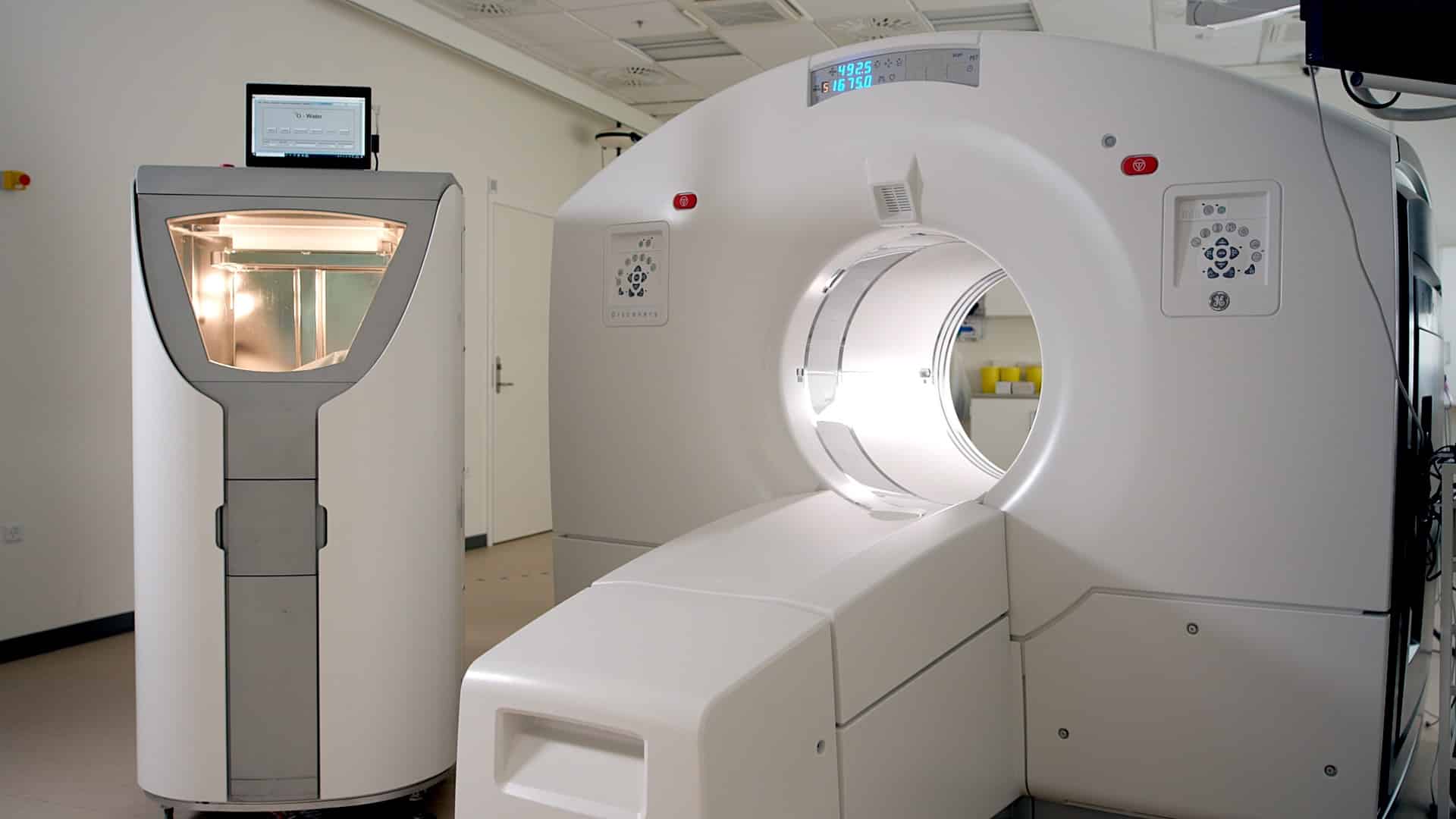
MedTrace’s innovation comprises of an automated chemistry system, which manufactures the gold standard perfusion tracer 15O-water under GMP compliance, as well as an analytical software platform that can both quantify and visualize blood flow. The hardware solves the challenge of 15O-water’s short half-life (122seconds) as the tracer is produced next to the patient and the PET scanner whereas the analytical software offers a novel combination of both quantitative flow data and flow visualization.
The EIC/H2020 grant co-finances the following work pakages project management, regulatory approval of the innovation in the EU, and paves the way for market access. The goal of the workpakages is to deliver the following:
Horizon 2020 is an EU funding programme, that provides research and innovation funding for multi-national collaboration projects and for individual researchers and supports SMEs with a special funding investment.
With help from the SME instrument, MedTrace is now able to make 15O-water practically available.
The assessment of myocardial blood flow is carried out by positron emission tomography (PET) using 15O-water, a radioactive variation of regular water as a tracer. However, the short half-life of 15O-water alongside difficulties in image analysis have prohibited its widespread clinical use.
Scientists of the EU-funded EU-CAD project are working on a new hardware and analytical software combination to aid clinicians all over the world with 15O-water PET images. The project aims to further develop the current prototype and validate it in patients before obtaining regulatory approval to operate in Europe. Given the implication of myocardial blood flow in coronary heart disease, a robust measurement approach will help patient outcomes.
For further information, please send an email with your inquiry to connect@medtrace.dk.