How we got started
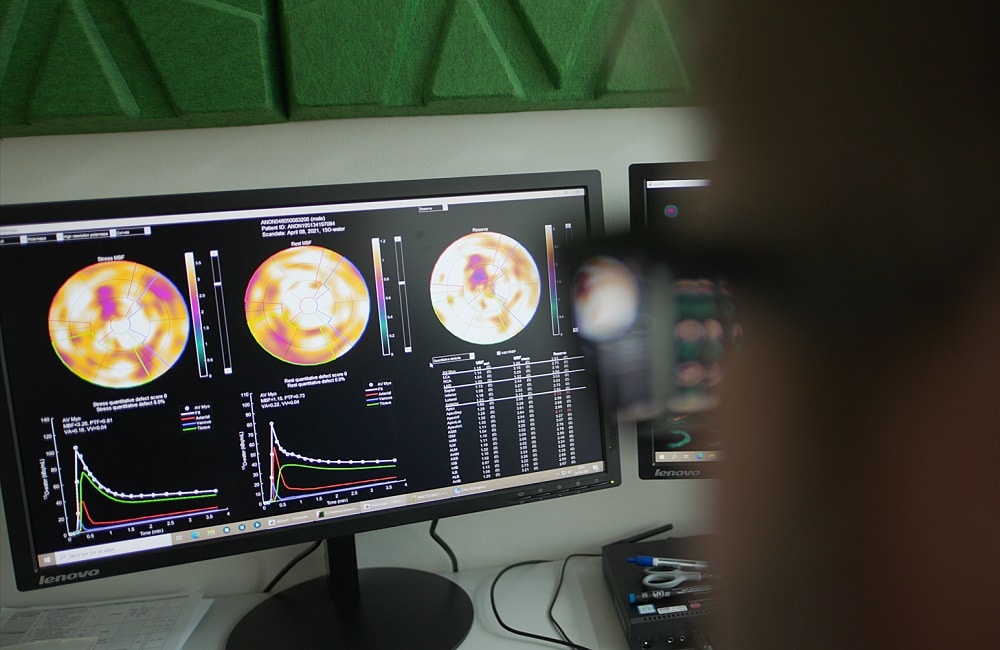
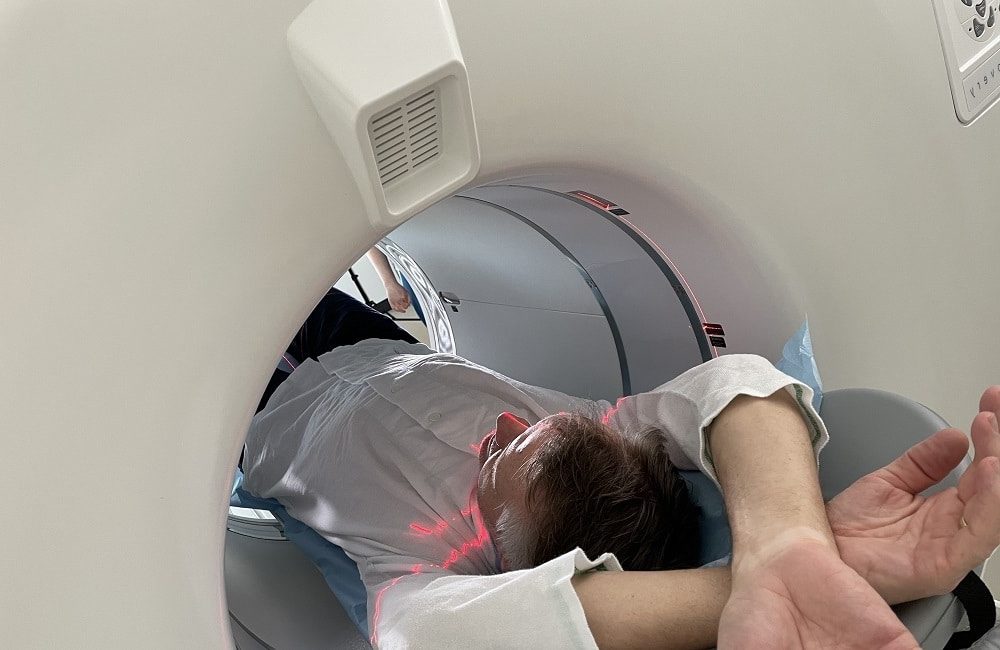
Our co-founder Rune Wiik Kristensen wondered why 15O-water is considered the gold standard3 for perfusion imaging, but it was not used routinely clinically. Rune, who had worked as a radiochemist in two of Denmark’s top hospitals, also wondered why cardiac PET (position emission tomography) wasn’t on the radar of most clinicians in the field, given the great success of oncological PET.
He learned that 15O-water was impractical in a clinical setting because it had such a short half-life, rendering the supply chain near impossible to scale up. Yet he also deduced that the short half-life, if controlled, would provide for reduced patient dosage and shorter scan times, increasing the feasibility of cardiac PET.
Rune decided to try to invent a device that would allow 15O-water to be produced right next to a PET scanner in a hospital, overcoming the short half-life and making 15O-water practically available.