MedTrace Announces IND Approval of RAPID-WATER Phase 3 Clinical Trial
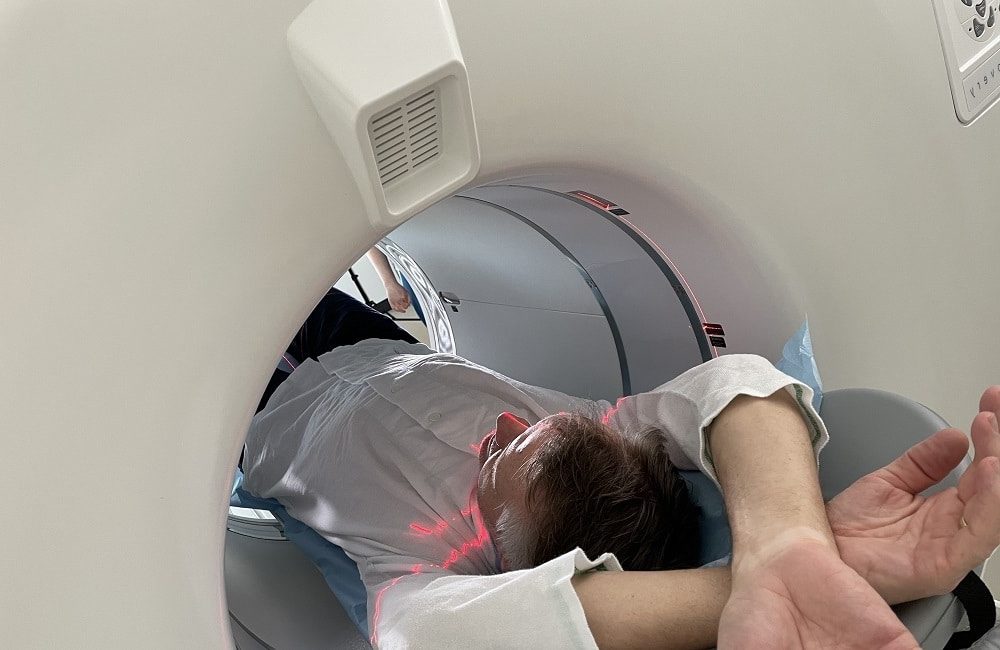
MedTrace Pharma Announces IND Approval with the Initiation of RAPID-WATER-FLOW Phase III Clinical Trial MedTrace Pharma A/S announced today the FDA approval of the company’s Investigational New Drug (IND) application and the approval to commence the RAPID-WATER-FLOW clinical trial to evaluate the use of 15O-water PET in diagnosing Coronary Artery Disease (CAD). The global trial […]
MedTrace Pharma Takes Step Towards CE Marking
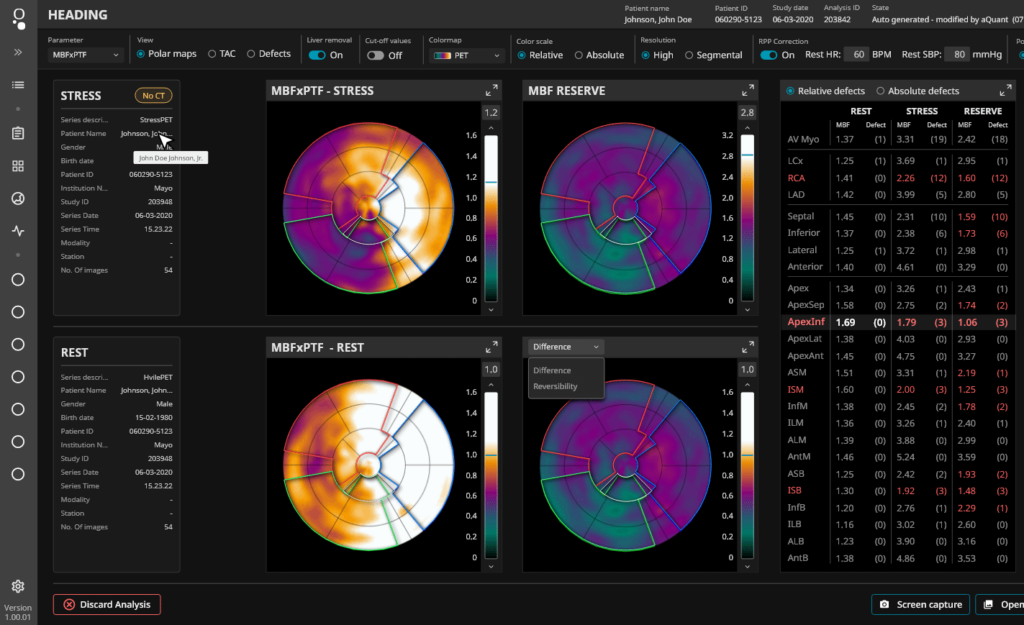
MedTrace Pharma Takes Step Towards CE Marking A notified body has signed a contract to review MedTrace Pharma’s aQuant software and patient kit products with an eye to CE marking. The notified body, BSI, is based in the Netherlands. “We’re encouraged that BSI has agreed to audit our products,” says Rune Wiik Kristensen of MedTrace […]
MedTrace Installs P3 System at Sahlgrenska University Hospital

MedTrace Installs P3 System at Sahlgrenska University Hospital MedTrace Pharma A/S has signed a contract with Sahlgrenska University Hospital in Gothenburg, Sweden for the installation of a new P3 production and infusion system providing up to 1000 doses of 15O-water per year for the next five years. The Gothenburg-based hospital, one of the largest in […]
Putting the Low Radiation Dose of 15O-Water in Perspective
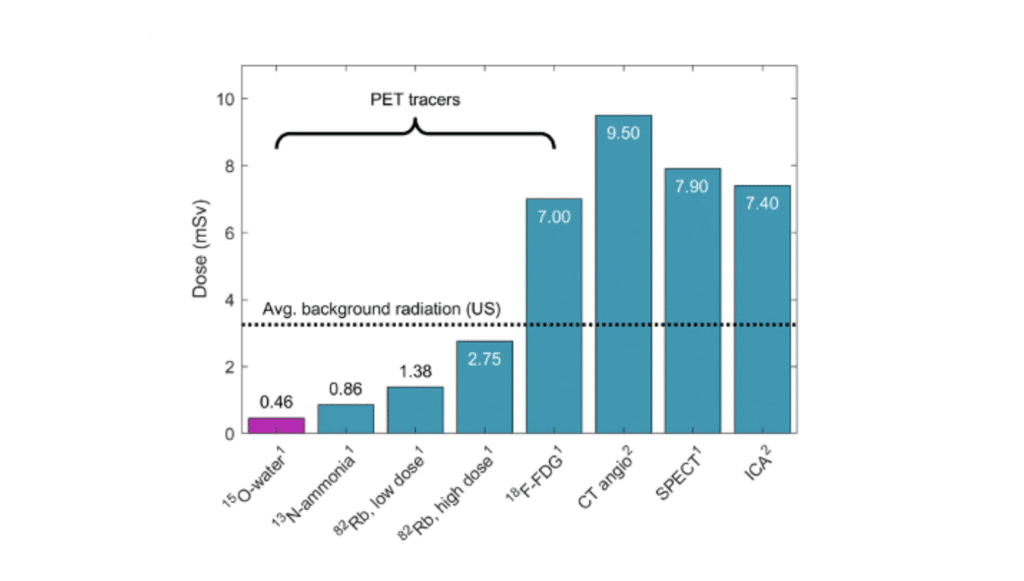
Putting the low radiation of 15O-water in perspective Tracers are radioactive compounds that doctors inject into patients to diagnose various diseases by, for example, following the flow of blood or use of sugar in organs. All tracers come with a radiation dose, but the dose of 15O-water is much lower than all other tracers used […]