MedTrace Hosts 15O-Water Symposium for Nuclear Medicine Professionals

MedTrace hosts 15O-water symposium for nuclear medicine professionals 15O-water: Ready for the Future. Vol. 2. The symposium, sponsored by MedTrace, will take place on September 11, 2023, in Vienna. Guests can expect to hear from some of the world’s leading experts when they present new research and share their first-hand experiences with 15O-water. MedTrace is […]
MedTrace Earns ISO 13485 Certification, Meeting Stringent Standards for Medical Devices

MedTrace Earns ISO 13485 Certification, Meeting Stringent Standards for Medical Devices MedTrace is now ISO 13485 certified for the company’s quality management system in medical devices. The first technical file for the medical devices Patient Kit and Patient Kit Extended has been submitted as class IIa products and is currently under review for CE marking. […]
First U.S. Patient Scanned in MedTrace’s 15O-water Phase 3 Trial

First U.S. Patient Scanned in MedTrace’s 15O-Water Phase 3 Trial The first U.S. patient in MedTrace’s phase 3 clinical trial has been dosed and scanned using the company’s automated manufacturing system for producing and dosing 15O-water. The trial seeks to evaluate the use of 15O-water PET in diagnosing coronary artery disease (CAD). It is the first phase […]
New Findings from Aarhus University Hospital Shed Light on How 15O-Water PET MPI May Benefit Patients with Bypass Surgery

New findings from Aarhus University Hospital shed light on how 15O-water PET MPI may benefit patients with bypass surgery 15O-water PET MPI may be a promising way to personalize treatment for patients with a history of bypass surgery who are still experiencing chest pain. That is the short version of the conclusion in a recent […]
Aarhus University Hospital Documents Frequent Extracardiac Findings on 15O-Water PET Myocardial Perfusion Imaging

Aarhus University Hospital document frequent extracardiac findings on 15O-water PET myocardial perfusion imaging From tumors and lung cancer to rib fractures and COVID-19 infiltrates. Researchers at Aarhus University Hospital document that 15O-water PET myocardial perfusion imaging (MPI) often reveals several extracardiac findings or, in other words, findings outside of the intended scope. In their article […]
First Oncology Patient Scanned with 15O-Water on MedTrace Equipment

First Oncology Patient Scanned with 15O-Water on MedTrace Equipment Osaka University Hospital has dosed and scanned the first subject with 15O-water in the university’s scientific study “Evaluation of blood flow of malignant tumor using 15O-water PET”. The study utilized 15O-water from MedTrace’s automated manufacturing system (P3 MT-100). “We are very proud that we have scanned […]
MedTrace Receives U.S. Patent for Diagnosing the Human Heart

MedTrace Receives U.S. Patent for Diagnosing the Human Heart The U.S. Patent and Trademark Office issued a patent to MedTrace for their method of diagnosing the human heart via 15O-water PET. The patented method is the foundation of the company’s software aQuant, currently under development. Hendrik “Hans” Harms, PhD and Senior Scientist at MedTrace, and Jens […]
15O-Water Myocardial Perfusion Imaging: A Practical Approach to Simplifying the Gold Standard for Approved Clinical Use
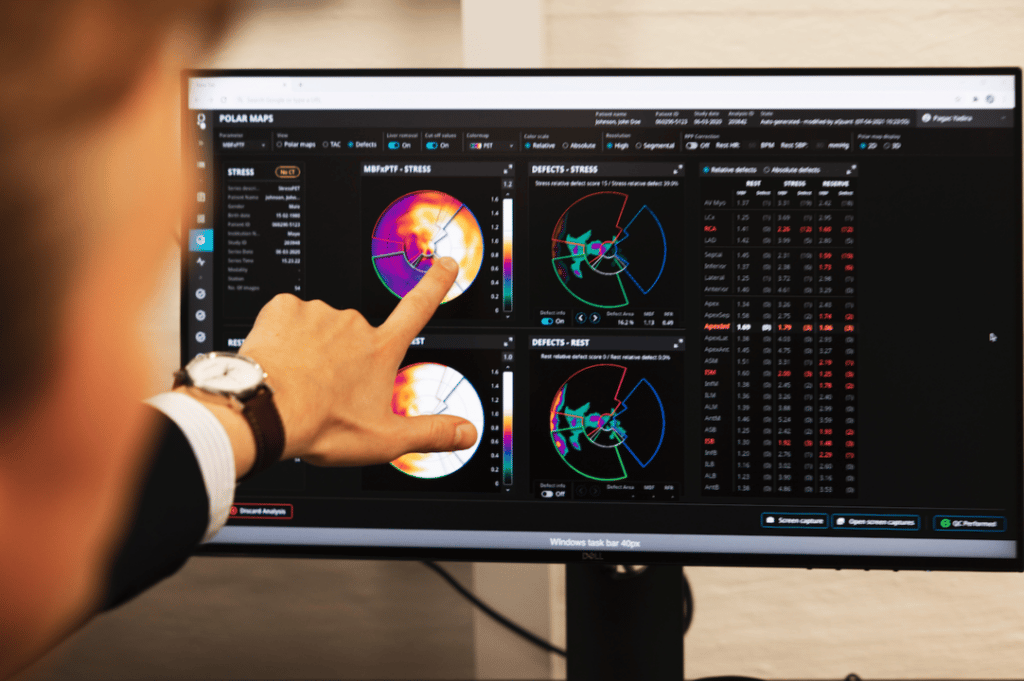
15O-water myocardial perfusion imaging: a practical approach to simplifying the gold standard for approved clinical use Mark Lubberink, PhD, Scientific Advisor to MedTrace & Hendrik “Hans” Harms, PhD, Senior Scientist at MedTrace, are contributing to the November issue of Society of Nuclear Medicine and Molecular Imaging (SNMMI)’s Value Initiative Newsletter. Lubberink & Harms elaborate on the challenges of […]
MedTrace Gathered +100 Doctors and Scientists of Nuclear Medicine for the Company’s Symposium on the Future of 15O-water

MedTrace Gathered +100 Doctors and Scientists of Nuclear Medicine for the Company’s Symposium on the Future of 15O-Water On October 17, 2022, MedTrace hosted a discussion on the future of 15O-water in conjunction with the 35th annual congress of the European Association of Nuclear Medicine in Barcelona. The event was the first symposium held by […]
MedTrace Announces First Subject Scanned in Phase 3 Clinical Trial
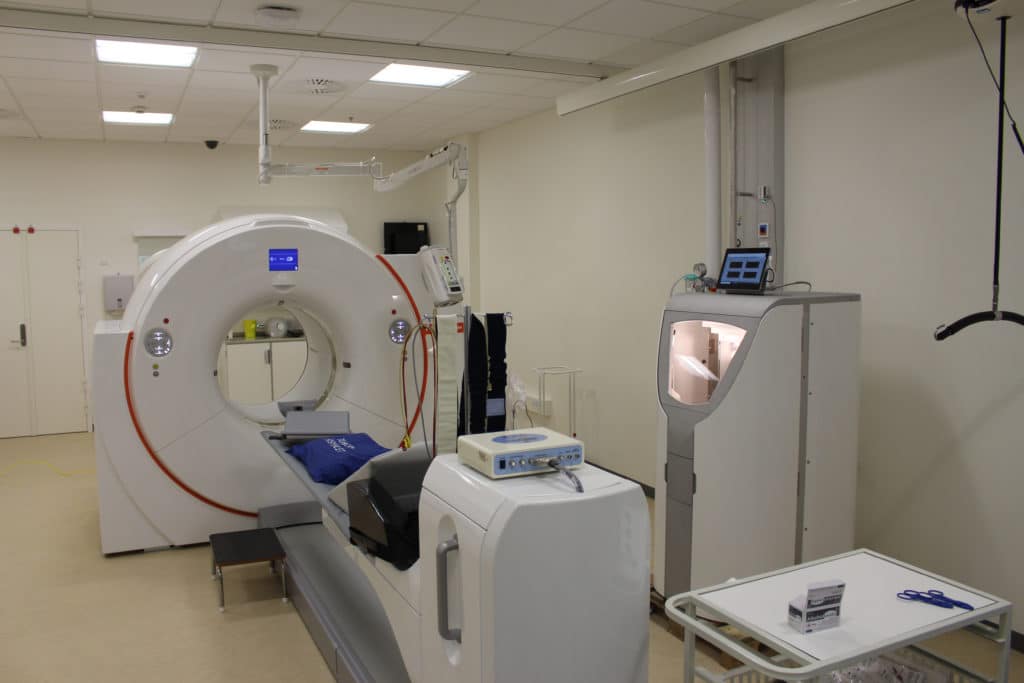
MedTrace Pharma Announces First Subject Scanned in the Company’s RAPID-WATER-FLOW Phase 3 Clinical Trial First Subject Scanned in RAPID-WATER-FLOW Trial at Aarhus University Hospital, Denmark Multiple Sites Involved Across the United States, Denmark, Sweden, and The Netherlands The Global Trial is Expected to Continue for 12 Months MedTrace Pharma A/S, a pharma and device company […]